-
Contents
-
Table of Contents
-
Troubleshooting
-
Bookmarks
Quick Links
USER’S GUIDE
Mark 5 Nuvo (Std)
LITE
OXYGEN CONCENTRATOR
[Original language is English ]
Federal Law (US) restricts this device to
sale or use by, or on the order of, a licensed
physician. This oxygen concentrator should
be used only under the supervision of a
licensed physician.
I.
DESCRIPTION ……………………………………………..2
II.
III.1. Cleaning ………………………………………………………….4
III.3. Maintenance…………………………………………………….4
: ON (power switched on)
: Off (power switched off).
: Type B device
: Class II protection
: Do not expose to open ames.
2010-8400 Rev F
Danger: Do not smoke when using oxygen
CONTENTS
IV.
IV.7. Standards…………………………………………………………6
GLOSSARY OF SYMBOLS
: Do not use oil or grease.
: Technical information.
: Consult the accompanying documents.
: Keep in the vertical position.
: Fragile — handle with care.
May 2011
or when near this device.
1
Summary of Contents for Nidek Medical Mark 5 Nuvo LITE
-
Contents
-
Table of Contents
-
Troubleshooting
-
Bookmarks
Quick Links
USER’S GUIDE
Mark 5 Nuvo (Std)
LITE
OXYGEN CONCENTRATOR
[Original language is English ]
Federal Law (US) restricts this device to
sale or use by, or on the order of, a licensed
physician. This oxygen concentrator should
be used only under the supervision of a
licensed physician.
I.
DESCRIPTION ……………………………………………..2
II.
III.1. Cleaning ………………………………………………………….4
III.3. Maintenance…………………………………………………….4
: ON (power switched on)
: Off (power switched off).
: Type B device
: Class II protection
: Do not expose to open ames.
2010-8400 Rev F
Danger: Do not smoke when using oxygen
CONTENTS
IV.
IV.7. Standards…………………………………………………………6
GLOSSARY OF SYMBOLS
: Do not use oil or grease.
: Technical information.
: Consult the accompanying documents.
: Keep in the vertical position.
: Fragile — handle with care.
May 2011
or when near this device.
1
Summary of Contents for Nidek Medical Mark 5 Nuvo LITE
Быстрая Доставка
Курьерская доставка заказов по всей России
- до Дверей
- до Терминала
Оплата при Получении
(наложенный платеж)
Оплата наличными или картой курьеру, после проверки соответствия, комплектации, работоспособности
Top 5 Товаров
Новости / Информация
Мы в социальных сетях

Эта модель доступна в аренду по Москве и Области
-
Описание
-
Устройство Mark 5 Nuvo Lite из линейки товаров американского бренда Nidek оптимально подойдет для оксигенотерапии на дому. Благодаря данному прибору лечение кислородной недостаточности является максимально простым и удобным для пациента. Преимуществом данной модели является минимальный вес, составляющий всего 13,6 кг. Корпус устройства снабжен ручкой и колесами, поэтому его перемещение не представляет сложности даже для пациентов зрелого возраста или людей с ограниченной подвижностью.
Компактный прибор занимает мало места, поэтому Mark 5 Nuvo Lite без труда устанавливается не только в помещении, но и в салоне медицинского транспорта. Аппарат отличается простотой настройки, а уровень шума при работе не создает неудобств. При этом модель является очень производительной, благодаря чему может успешно использоваться для профилактического лечения и оказания срочной медицинской помощи.Экономичность и легкость в обслуживании
Достоинством концентратора Mark 5 Nuvo Lite является простота сервисного обслуживания. При регулярном применении прибора замена фильтра предварительной очистки производится всего один раз в год.
В целях повышения эффективности кислородной терапии устройство оборудовано трехступенчатой системой фильтрации воздуха, благодаря чему происходит не только повышение содержания кислорода, но и бактериальная очистка воздуха.
Также Mark 5 Nuvo Lite снабжен диагностическим модулем для непрерывного контроля содержания кислорода. При изменении показателей срабатывает световой и звуковой индикатор.Применение устройства
Концентратор кислорода «Марк 5 НУВО Лайт» компактный и высокопроизводительный прибор.
«Марк 5 Нуво Лайт» используется очень широко:- Для организации лечения на дому с целью продолжительной кислородной терапии.
- В медицинских учреждениях, при проведении мер неотложной помощи.
- При проведении процедур профилактической кислородотерапии.
- Предназначен для длительной кислородотерапии на дому, в больничных и поликлинических условиях.
- Расходомер с регулятором потока кислорода от 0, 125 до 5 л/мин. (12 положений регулятора потока)
- При максимальном потоке 5 л/мин концентрация кислорода составляет более 90%.
- Круглосуточная работа в течение 24 часов.
- Звуковые предупреждения и оптические индикаторы.
- Счетчик проработанного времени (в часах).
В спортивных клубах и учреждениях санаторно-курортного типа прибор можно использовать для приготовления кислородных коктейлей.
ТЕХНИЧЕСКИЕ ХАРАКТЕРИСТИКИ Марк 5 НУВО Лайт
Ресурс до 30 000 часов Электропитание 220/230В ~50Гц 1,55А Поток кислорода от 0,125 до 5 литров в минуту (л/мин) Концентрация кислорода в процентах от 0,125 до 5 л/мин 90% (+6,5%/3%) Максимальное давление кислорода на выходе 50,0 кПа (7,0 psig) Подсчет проработанного времени Счетчик времени (в часах) Уровень шума менее < 45 ДБ Мощность максимум 290 Вт Принадлежности Увлажнитель кислорода
Канюля носовая с трубкой
Маска кислородная с трубкойЭксплуатация при температуре и влажности от 5°С до 40°С, от 40% до 95% Условия хранения Температура: от 40°С до 70°С
Влажность: от 10% до 100%Вес Нетто 14,5 кг Вес Брутто 16 кг Упаковка Марк 5 нуво лайт Картонная коробка с полиуретановыми вставками Габаритные размеры 585 мм (в) х 360 мм (ш) х 230 мм (г) Габаритные размеры упаковки 660 мм (в) х 480 мм (ш) х 340 мм (г) Соответствие европейским директивам и стандартам 93/42 EEC, IEC606012, EN60601+A1+A2,
ISO8359:1996, ISO9001: 2000/ISO13485:2003Классификация устройства класс II, тип B Гарантия 3 года -
Комплектация
-
Комплектация Nidek Марк 5 нуво лайт
Концентратор кислорода Nidek Mark 5 Nuvo Lite 1 шт
Удлинительная кислородная трубка 10 м. 1шт.
Маска кислородная 1шт.
Канюля кислородная, трубка 1.8м. 1 шт
Увлажнитель кислорода 1 шт
Фильтр грубой очистки 1 шт
Фильр тонкой очитки, оригинал для Mark 5 Nuvo Lite 1 шт
Инструкция по эксплуатации 1 шт
Гарантийный талон 1 шт
-
Документы
-
Инструкция по эксплуатации
-
Руководство по эксплуатации mark 5 nuvo lite инструкция
-
Техзадание
-
Техническое задание на Кислородный концентратор Mark 5 nuvo lite
Скачать в формате .docx
Комплект поставки:
Концентратор кислорода 1 шт.
Удлинительная кислородная трубка 10 м. 1шт.
Маска кислородная 1шт.
Канюля кислородная, трубка 1.8м. 1 шт.
Увлажнитель кислорода 1 шт.
Фильтр грубой очистки 1 шт.
Фильтр тонкой очитки 1 шт.
Инструкция по эксплуатации 1 шт.
Гарантийный талон 1 шт.Наименование товара, товарный знак Концентратор кислорода Mark 5 Nuvo Lite Наименование страны происхождения товара Соединенные Штаты Америки, ОКСМ: USA, 840 Описание требований Наличие функции или величина параметра Назначение Для длительной кислородотерапии Счетчик проработанного времени (в часах) наличие Звуковые предупреждения, оптические индикаторы наличие Оптические индикаторы наличие Уровень шума 37 Дб Максимальное давление кислорода на выходе 50,0 кПа Поток кислорода в диапазоне от 0,125 л/мин до 5 л/мин (12 позиций переключателя) Концентрация кислорода 90-95% Электропитание 220В, 50Гц, 1,55А Потребляемая электрическая мощность 290 Вт Габаритные размеры 580 мм (высота) х 350 мм (ширина) х 220 мм (глубина) Размеры в упаковке 660 мм (в) х 480 мм (ш) х 340 мм (г) Масса, нетто 13,6 кг Масса, брутто 16 кг Упаковка Картонная коробка Колесики для передвижения 4 шт. вмонтированы в корпус Классификация устройства класс II, тип B Гарантия производителя 3 года

Единиц товара в коробке: 1
Этот концентратор приобретался на замену вышедшему из строя Атмунг 5лН. Сравнивать есть с чем, отмечу плюсы При той же производительности,1.Размеры в два раза меньше 2.Значительно легче по весу 3.Тише работает, со слов консультанта и информации из инструкции на марк 5 нуво лайт, в нем встроен газоанализатор который сигнализирует о снижении концентрации кислорода на выходе (пока не срабатывал), что крайне важно для человека напрямую зависящего от концентратора. Подключение и настройка проще простого, всё в комплекте. Прибор рекомендую! заказ №0147
Заказ 127. С огромной благодарностью и наилучшими пожеланиями!
Концентратор приобретался для больного ХОБЛ. С помощью консультантов, выбор пал именно на эту модель за счет небольшого веса, габаритов и удобства пользования. Всё управление сводится к включению и выключению, пожилой человек с легкостью справляется. Используем концентратор Марк 5 уже вторую неделю, никаких нареканий нет, всё работает отлично.В комплекте есть всё необходимое, ничего не нужно докупать. Сотрудников магазина благодарим за консультацию, предоставленную скидку и быструю доставку.
Нет равных !!!
09.07.2018 года заказал Кислородный концентратор Mark 5 Nuvo Lite производстра и Сборки США! с 10% скидкой получилось 47 610 рублей. То есть по цене Китайских тракторов типа Амед или Амунг. Пульсоксиметр подарили бесплатно, доставили в Истру, на следующий день 10.07.2018 года, доставка бесплатная!!! Что тоже очень порадовало. Концентратор кислорода Mark 5 Nuvo Lite за месяц непрерывной (24 часа в сутки) эксплуатации показал себя превосходно: очень легки, очень тихий (по уровню шума как домашний компьютер) и ,самое главное в моем случае, очень простой!!! (покупался для пожилого человека 82 года ХОБЛ). Ребятам из Air-med.ru отдельное Спасибо! За прекрасную слаженную работу и отличные условия для покупки (бесплатная и быстрая доставка, скидка и подарки, проверка концентратора при доставке и обучение по его использованию). Успехов и процветания!!!
Другие отзывы
Вы уже оставили отзыв об этом товаре. Спасибо!


Nidek Medical Products, Inc®
Mark5 Nuvo® [M5C5] Oxygen
Concentrator Service Manual
Nidek Medical Products, Inc. 3949 Valley East Industrial Drive Birmingham, Alabama 35217 USA
Telephone: (205) 856-7200 • 24-Hour Fax: (205) 856-0533
Nidek Medical is a trademark of Nidek Medical Products, Inc.
Mark5 Nuvo® and M5C5 are registered trademarks of Nidek Medical Products, Inc.
|
Page 2 of 59 |
2010-2225 Rev — B |

|
Table of Contents |
3-5 |
||
|
General Safety |
|||
|
Instructions |
|||
|
Production and Use of Oxygen |
6 |
||
|
Use and Maintenance of the Device |
6 |
||
|
Standards and Regulations |
6 |
||
|
Section 1.0 |
|||
|
Introduction |
|||
|
1.1 |
Home Service Provider Responsibility |
7 |
|
|
1.2 |
Important Notice and Symbol Explanations |
8 |
|
|
1.3 |
Functional Specifications |
9 |
|
|
Section 2.0 |
|||
|
Operational Check and Concentration Test |
|||
|
2.1 |
Description of Operation |
9 |
|
|
2.2 |
Operation Check |
10 |
|
|
2.3 |
Alarm System |
10 |
|
|
2.3.1 |
Battery Test |
11 |
|
|
2.3.2 Power Failure Alarm test |
11 |
||
|
2.4 |
Oxygen Concentration Test and Specification |
11 |
|
|
Section 3.0 |
|||
|
Patient Instructions |
|||
|
3.1 |
General Instructions |
12 |
|
|
3.2 |
Routine Maintenance by the Patient |
12 |
|
|
3.2.1 Cleaning the Cabinet Air Filter |
12 |
||
|
3.2.2 Checking the Alarm System Battery |
13 |
||
|
Section 4.0 |
|||
|
Home Service Provider Maintenance |
|||
|
4.1 |
Routine Maintenance |
13 |
|
|
4.1.1 |
Cabinet Air Filter |
13 |
|
|
4.1.2 Final Product Filter Replacement |
13 |
||
|
4.1.3 Inlet Air Filter Replacement |
14 |
||
|
4.1.4 |
Battery Replacement |
14 |
|
|
4.1.5 |
Wick Replacement |
14 |
|
|
4.1.6 |
Recording Maintenance |
15 |
|
|
4.2 |
Cleaning and Infection Control |
15 |
|
|
4.2.1 Preparing for New Patient Use |
15 |
||
|
Section 5.0 |
|||
|
Service |
|||
|
5.1 |
Components |
16 |
|
|
5.2 |
Cabinet Removal |
16 |
|
|
5.2.1 |
Removing Cabinet Back |
16 |
|
|
5.2.2 |
Removing Cabinet Front |
16 |
|
|
5.2.3 |
Removing Control Panel |
16 |
|
|
5.2.4 |
Main Structure |
16 |
|
2010-2225 Rev — B |
Page 3 of 59 |

|
5.2.5 |
Caster Replacement |
17 |
|
|
5.3 |
Compressor |
17 |
|
|
5.3.1 |
Compressor Replacement |
18 |
|
|
5.3.2 |
Capacitor Replacement |
19 |
|
|
5.4 |
Process Control Valve |
19 |
|
|
5.5 |
Sieve Bed Replacement |
19 |
|
|
5.5.1 |
Sieve Bed Removal |
20 |
|
|
5.5.2 |
Sieve Bed Installation |
20 |
|
|
5.6 |
Cabinet Fan Replacement |
20 |
|
|
5.7 |
Circuit Board Replacement |
21 |
|
|
5.7.1 |
Circuit Board Removal |
21 |
|
|
5.7.2 |
Circuit Board Installation |
22 |
|
|
5.8 |
Product Regulator Check and Setting |
22 |
|
|
5.8.1 Product Regulator Cleaning or Rebuilding |
22 |
||
|
5.9 |
Pressure Switch Replacement |
23 |
|
|
5.10 |
Circuit Breaker Replacement |
23 |
|
|
5.10.1 Circuit Breaker Removal |
23 |
||
|
5.10.2 Circuit Breaker Installation |
23 |
||
|
5.11 |
I/0 (ON/OFF) Power Switch Replacement |
23 |
|
|
5.11.1 I/0 (ON/OFF) Power Switch Removal |
23 |
||
|
5.11.2 I/0 (ON/OFF) Power Switch Installation |
24 |
||
|
5.12 |
Buzzer Replacement |
24 |
|
|
5.13 |
Hour Meter Replacement |
24 |
|
|
5.14 |
Flow Meter Replacement |
24 |
|
|
5.14.1 Flow Meter Removal |
24 |
||
|
5.14.2 Flow Meter Installation |
24 |
||
|
5.15 |
Power Cord Replacement |
24 |
Section 6.0
Troubleshooting
|
6.1 |
Air Pressure Test (P1) |
25 |
|
6.1.1 High Air Pressure |
25 |
|
|
6.1.2 Low Air Pressure |
26 |
|
|
6.2 |
Product Pressure Test (P2) |
26 |
|
6.2.1 High Product Pressure |
26 |
|
|
6.2.2 Low Product Pressure |
27 |
|
|
6.3 |
General Troubleshooting |
27 |
|
6.4 |
Troubleshooting Chart |
28-32 |
|
6.5 |
Tool Kit and Pressure Test Gauge |
33 |
|
Page 4 of 59 |
2010-2225 Rev — B |

Appendices
|
Exploded |
A1: Base Assembly |
34 |
|
Drawings |
A1.1: Base Assembly |
35 |
|
A2: Main Structure Assembly – Front View |
36 |
|
|
A3: Main Structure Assembly – Rear View |
37 |
|
|
A4: Regulator Assembly |
38 |
|
|
A5: Compressor Assembly |
39 |
|
|
A5.1: Compressor Assembly |
40 |
|
|
A6: Control Panel Assembly; OCSI Unit — Front |
41 |
|
|
A7: Control Panel Assembly; OCSI Unit — Rear |
42 |
|
|
A6.1: Control Panel Assembly; OCSI Unit — Front |
43 |
|
|
A7.1: Control Panel Assembly; OCSI Unit — Rear |
44 |
|
|
A8: Control Panel Assembly; Non OCSI Unit — |
45 |
|
|
Front |
||
|
A9: Control Panel Assembly; Non OCSI Unit – |
46 |
|
|
Rear |
||
|
A10: Air Tank Assembly |
47 |
|
|
A10.1: Air Tank Assembly |
48 |
|
|
A11: Front Cabinet Assembly |
49 |
|
|
A11.1: Front Cabinet Assembly |
50 |
|
|
A12: Rear Cabinet Assembly |
51 |
|
|
A12: Rear Cabinet Assembly |
52 |
|
|
A13: Module Assembly |
53 |
|
|
Parts Listing |
A14: Replacement Parts Listing |
54-57 |
|
Maintenance |
A15: Maintenance Log |
58-59 |
|
Record |
|
2010-2225 Rev — B |
Page 5 of 59 |
General Safety Instructions
Production and use of oxygen
Oxygen is not a flammable gas, but accelerates the combustion of materials. To prevent fire risks, the MARK5 NUVO should be kept away from flames, incandescent sources or sources of heat (cigarettes) and combustible products such as oil, grease, solvents, aerosols, etc.
Do not use in an explosive atmosphere.
Prevent oxygen from accumulating on upholstered seats or any other fabric. If the concentrator operates without being administered to a patient, locate it so that the flow of gas generated is dissipated into the air.
Locate the equipment in a free space (filter to the rear and below) which is well ventilated and free of fumes or atmospheric pollution.
Use and Maintenance of the Device
Use the electric cable provided and check that the voltage of the mains socket used complies with the electrical characteristics of the appliance indicated on the manufacturers plate on the rear of the appliance.
Do not use an extension cord or multiple sockets which can create sparks and therefore pose a fire risk.
Use of the MARK5 NUVO must be restricted solely to oxygen therapy on medical prescription in compliance with the daily rate and duration.
Use in other circumstances may represent a hazard to patient health.
Do not use in a specifically magnetic environment (MRI, etc.).
The MARK5 Nuvo has an audible alarm intended to warn the user of any problems. The user must determine the maximum distance away from the Nuvo based on the on the sound levels in the environment, to ensure that the alarm is always audible.
Standards & Regulations
In compliance with UL60601-1 [EN60601-1] (para 6.82.b):
“The manufacturer, assembler, installer or importer are not considered to be responsible for consequences or the safety, reliability and characteristics of a device unless,
● the assembly, extensions, adjustments modifications or repairs have been performed by persons authorized by the manufacturer,
Page 6 of 59 2010-2225 Rev — B
●the electrical installation of the corresponding premises complies with appropriate regulations and codes,
●the device is used in accordance with the instructions for its use.
If the replacement parts used for periodic servicing by an approved technician do not comply with the manufacturer’s specifications, the manufacturer is absolved of all liability in the event of an incident.
Do not open the equipment when it is powered on: risk of electrocution.
This device complies with the requirements of the FDA Quality System Regulation and EU Directive 93/42/EEC, but its operation may be affected by the use in the surrounding area of appliances such as diathermy, high frequency electro-surgical instruments, defibrillators, short wave treatment appliances, cell-phones, CB devices and other portables, microwave ovens, induction hot plates or remote control toys, and more generally, by electromagnetic interference exceeding the levels specified in standard IEC(EN) 60601-1-2.
1.0Introduction
1.1Home Service Provider Responsibility
All Home Service Providers of the Nidek Medical Mark5 Nuvo® Oxygen Concentrator must assume responsibilities for handling, operational check-out, patient instruction, and maintenance. These responsibilities are outlined below and throughout this manual.
WARNING
Mark5 Nuvo units must not be used for or with any life-supporting or life sustaining applications. Patients unable to communicate discomfort while using this device may require additional monitoring. Advise patients to immediately notify their Home Service Provider(s) and/or physician(s) in case of an alarm or any discomfort.
As a Home Service Provider, you must do all of the following:
●Inspect the condition of each Mark5 Nuvo® unit immediately upon delivery to your business location. Note any sign of damage, external or internal, on the delivery receipt, and report it directly to both the freight company and Nidek Medical Products, Inc. immediately.
●Check the operation of each Mark5 Nuvo® before delivery to a patient.
Always operate the unit for a reasonable length of time and check that the oxygen concentration level is within specifications as referred to in Section 2.4. Test the battery and power disconnect alarm as described in Section 2.3 of this manual.
|
2010-2225 Rev — B |
Page 7 of 59 |

●Deliver Mark5 Nuvo® units only to patients authorized by a physician’s prescription. The Mark5 Nuvo® must not be used as a life-supporting or life sustaining device. A backup supply of oxygen must be available.
●Instruct patients how to use the Mark5 Nuvo® in conjunction with the
Patient Manual.
●Instruct patients to notify their physicians and/or Home Service Providers if they experience any signs of discomfort.
●Instruct each patient how to perform routine maintenance of the cabinet air filter and how to check the alarm system battery. (Refer to Section 3.2.)
Be available to service each patient at any time. Maintain the Mark5 Nuvo® in accordance with Section 4.0.
Repair components and replace parts only as outlined in this manual. Use only Nidek Medical parts for replacement in Mark5 Nuvo® Oxygen Concentrators.
●Refer to the Mark5 Nuvo® Product Warranty if parts replacement is required within the warranty period.
1.2Important Notice and Symbol Explanations
As you read the manual, pay special attention to the WARNING, CAUTION, and NOTE messages. They identify safety guidelines or other important information as follows:
|
WARNING: |
Describes a hazard or unsafe practice that can result |
|
|
in severe bodily injury or death. |
||
|
CAUTION: |
Describes a hazard or unsafe practice that can result |
|
|
in minor bodily injury or property damage. |
||
|
NOTE: |
Provides information important enough to emphasize |
|
|
or repeat. |
||
The following harmonized symbols (pictograms), used for non-English language countries, will be located on the exterior of the Mark5 Nuvo® unit:
Read the accompanying documents; particularly the patient operating guide.
Store, ship and use the device in an upright condition.
No smoking within five feet of this device, oxygen-carrying tubing, or accessories.
Indicates an alarm signal.
|
Page 8 of 59 |
2010-2225 Rev — B |

Do not use any oil or grease on or near the device
1.3Functional Specifications
Dimensions: 15.5 in. long, 15.6 in. wide, 27.8 in. tall (394 mm long, 396 mm wide, 706 mm high)
|
Weight: |
50 to 54 Ib (22.5 to 24.5 kg) depending on sound attenuation; |
|
|
Electrical |
120 VAC, 60 Hz, 4 amps, 410 watts |
|
|
Requirements: |
220 VAC, 50 Hz, 2 amps, 420 watts |
|
|
Capacity: |
5 liters per minute at 90% oxygen |
|
|
(Based on 70°F [21°C] at sea level) |
||
|
Accuracy: |
Flow meter ±5% full scale. (Based on pre-calibrated analysis.) |
|
|
Concentration: |
1 — 3 liters per minute at 95% + 0.5 / — 3% |
|
|
4 liters per minute at 92% + 3.5 / — 3% |
||
|
5 liters per minute at 90% + 5.5 / — 3% |
||
|
Response Time: |
Acceptable concentration is normally achieved in about |
|
|
90 seconds; allow 5 minutes to attain full concentration. |
||
|
Positioning: |
Operate the unit in an upright position, maintaining at |
|
|
least six inches of open space on all sides for ventilation. |
2.0Operational Check and Concentration Test
2.1Description of Operation
Air enters the Mark5 Nuvo® Oxygen Concentrator through an external cooling air intake filter. This filtered air enters the compressor via a suction resonator and fine filter, which quiets the suction sounds made by the compressor. Pressurized air then exits the compressor and passes through a heat exchanger into an air tank. The heat exchanger reduces the temperature of the compressed air and the air tank stores air when feed is interrupted. Next, a rotary poppet feed valve directs the air into one of two sieve beds that contain molecular sieve. The special characteristic property of molecular sieve is that it physically attracts (adsorbs) nitrogen when air passes through this material, thus enabling the production of high purity oxygen.
There are two sieve beds or adsorbent columns; while one produces high purity oxygen, the other is purged of the nitrogen it adsorbed (collected) while it was producing oxygen. Each column produces oxygen for approximately four seconds and delivers it to the product storage volume in the end of the column. Oxygen exits the adsorbent column through a pressure regulator, flow control valve, flow meter, and final product filter. The flow control valve, which is part of the flow meter, controls the flow rate of oxygen delivered to the patient. The Mark5 Nuvo® unit 2010-2225 Rev — B Page 9 of 59
delivers up to 95% oxygen concentration at flow rates from 1 to 5 l/min. The remaining constituents of the product gas stream are nitrogen and argon, both of which are part of the air we breathe, are inert and are completely safe.
2.2Operational Check
Nidek Medical runs each device through a burn in period and tests every Mark5 Nuvo® Oxygen Concentrator thoroughly after manufacture before releasing for shipment. As the home service provider, it is your responsibility to perform the following test to ensure that no damage occurred in shipping or handling.
1.Open and inspect all concentrator cartons upon receipt. Unpack each unit and remove it from its carton. Inspect the unit itself for damage. If the exterior of the carton is damaged, or the unit itself is damaged, note it on the freight bill signed by the driver.
●Before plugging the unit into the wall outlet, actuate the I/0
(ON/OFF) switch to the I (ON) position and note that the audible alarm sounds continuously. (See Section 2.3). Move the switch to the 0 (OFF) position.
2.Plug in the power cord of the unit, and set the I/0 (ON/OFF) switch to the I (ON) position. Check to see that the following occurs:
●The compressor runs, listen for the sound.
●Exhaust air flows out of the bottom of the unit.
●OPTIONAL for Units Equipped with Oxygen Concentration Status
Indicator (OCSI): The OCSI green light remains off until the oxygen concentration reaches 85% ± 3% (82% ± 2% for 50 Hz units) (approximately two minutes).
3.Turn the flow meter adjustment knob counterclockwise until it stops (wide open). The flow meter should register in excess of 5.5 liters/min. If not, refer to Section 5.8 to adjust the product regulator.
4 Perform an oxygen concentration test, as described in Section 2.4.
2.3Alarm System
The Mark5 Nuvo® Oxygen Concentrator is equipped with a battery-powered alarm system, which sounds a continuous and loud alarm when a power failure occurs or when one or more cycle variables are not within specification. It sounds an alarm if the high or low pressure indicators are activated or if the optional OCSI detects lower than predetermined levels of oxygen concentration. The alarm remains on until you correct the alarm condition or you set the I/0 (ON/OFF) switch to the 0 (OFF) position. Refer to Section 6.0 for a list of probable alarm causes,
|
Page 10 of 59 |
2010-2225 Rev — B |
2.3.1 Battery Test
The Mark5 Nuvo® battery can be checked by observing that the continuous alarm sounds when the I/0 (ON/OFF) switch is moved to the I (ON) position when the unit is unplugged from the wall outlet.
2.3.2 Power Failure Alarm Test
To test the power failure alarm, perform the following actions:
Unplug the power cord from the wall outlet, and set the I/0 (ON/OFF) switch to the I (ON) position.
This should immediately activate the audible alarm. If it does not, refer to the troubleshooting chart in Section 6.0 of this manual.
2.4Oxygen Concentration Test and Specification
To ensure that the output of oxygen from the device is within specification, you must perform an oxygen concentration test. Test the unit upon delivery to a patient and at periodic intervals. Home Service Providers, based on their expertise and documentation, may establish and implement their own plans for checking oxygen concentration. The interval established may be longer or shorter than 90 days, which is Nidek Medical’s default time period for providers who do not choose to establish their own plan.
1.If an oxygen humidifier bottle is used, remove it from the oxygen outlet.
2.Connect a calibrated oxygen concentration analyzer to the oxygen outlet.
3.Set the I/0 (ON/OFF) power switch to the I (ON) position. (It takes approximately five minutes for the oxygen concentration to stabilize.) Take oxygen concentration readings over a period of several minutes to reduce any cyclic variations
4.Verify that the product flow rate delivered by the unit matches the patient’s prescription and does not exceed the capacity of the unit.
5.Disconnect the oxygen analyzer, and reconnect the humidifier bottle (if used) and any other equipment / accessories that may be required.
6.Adjust the flow meter adjustment knob to the prescribed flow rate.
NOTE
Do not measure oxygen concentration output after the product stream passes through a humidifier bottle, erroneous readings will result and your oxygen concentration measuring device might be damaged.
|
2010-2225 Rev — B |
Page 11 of 59 |

Nidek Medical Mark5 Nuvo® Concentration Specifications
|
Liter Flow |
Specification |
Within Specification |
|
1 to 3 l/min |
95% + 0.5 / — 3% |
92% or higher |
|
4 l/min |
92% + 3.5 / — 3% |
89% or higher |
|
5 l/min |
90% + 5.5 / — 3% |
87% or higher |
3.0Patient Instructions
3.1General Instructions
It is important that patients thoroughly understand how to operate the Nidek Medical Mark5 Nuvo® unit. This enables proper treatment as prescribed by a qualified, licensed physician. You must explain that the purpose of this therapy is to alleviate symptoms. If patients experience any discomfort or the unit alarms, they must notify their Home Service Provider and/or physician immediately. You, as the Home Service Provider, are responsible to see that each patient receives the Patient Manual. Explain each step in the operation of the unit to the patient in reference to this manual.
3.2Routine Maintenance by the Patient
To ensure accurate output and efficient operation of the unit, the patient must perform two simple routine maintenance tasks:
•Clean the cabinet air filter
•Check the alarm system battery
3.2.1Cleaning the Cabinet Air Filter
NOTE:
The patient must clean this filter weekly, as described below. The filter may require daily cleaning if the Mark5 Nuvo® unit operates in a harsh environment such as a house heated by wood, kerosene, or oil, or one with excessive cigarette smoke.
1Remove the dirty cabinet air filter from the back of the Mark5 Nuvo® unit.
2Wash the dirty filter in warm water with household detergent, and rinse.
3Use a soft absorbent towel to remove excess water.
4Reinstall the clean air filter on the grille in the back of the unit.
|
Page 12 of 59 |
2010-2225 Rev — B |
3.2.2 Checking the Alarm System Battery
See Procedure described in Paragraph 2.3.1
4.0Home Service Provider Maintenance
4.1Routine Maintenance
The Mark5 Nuvo® unit has two filters and a 9-volt battery that require scheduled maintenance and replacement.
To ensure that the output of oxygen from the unit is within specification, you must perform an oxygen concentration test. Test the unit upon delivery to a patient and at periodic intervals. Home Service Providers, based on their expertise and documentation, may establish and implement their own practices for checking oxygen concentration. Consult Nidek Medical’s Service and Maintenance Log (A- 15) for the recommended intervals for testing.
Nidek Medical does not require preventive maintenance on the concentrator. You do not need to perform any maintenance as long as the Mark5 Nuvo® unit remains within specifications at the prescribed flow rate. (Refer to Section 2.4)
Cabinet Air Filter
The external cabinet air filter is located on the back of the unit, You can easily remove it by hand. Instruct the patient to clean this filter weekly. (Refer to Section 3.2.1.)
NOTE
The filter may require more frequent cleaning if the Mark5 Nuvo® unit operates in a harsh environment such as a house heated by wood, kerosene, or oil, or one with excessive cooking, cigarette smoke or atmospheric dust.
4.1.2 Final Product Filter Replacement
The final product filter does not require periodic replacement; it needs to be replaced only if it restricts oxygen flow. It is suggested that it be replaced whenever the sieve module is repaired or replaced and after the compressor is rebuilt.
1.Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2.Remove the cabinet back to locate the final product filter.
NOTE: Observe the position of the filter before removal.
3.Separate the silicone tubing from both sides of the filter.
4.Install the new filter with the inlet side in the same position as before. Push the tubing together so that it overlaps the barbs of the final product filter
|
2010-2225 Rev — B |
Page 13 of 59 |
connections.
5.Record information about the final product filter replacement in Appendix 15 of this manual and online at www.nidekmedical.com under the ‘Maintenance Log’ tab.
6.Reinstall the cabinet back.
4.1.3 Inlet Air Filter Replacement
The inlet air filter requires inspection at each patient visit. The filter should be replaced annually, or more often depending on environment.
1.Set the unit I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2.Remove the rear cabinet access panel to locate the inlet air filter.
3.Remove filter from the unit, and replace with a new filter.
4.Record information about the filter replacement in Appendix 15 of this manual and online at www.nidekmedical.com under the ‘Maintenance Log’ tab.
5.Reinstall the access panel.
4.1.4 Battery Replacement
Each time the standard Mark5 Nuvo® unit is turned on, the alarm must sound loudly for approximately five seconds to indicate a good battery. An alarm that does anything other than sound loudly for five seconds indicates a weak battery and requires replacement. For units with OCSI, actuate the switch with the power cord unplugged to test the battery.
To replace the battery, take the following steps:
1.Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2.Remove the access panel in the cabinet back.
3.Pull the battery from the mounting slot and remove the connection wires.
4.Attach the new battery to the connector and reinsert it into the mounting slot.
5.Set the I/0 (ON/OFF) switch to the I (ON) position to test the alarm.
6.Record the battery replacement information in Appendix 15 of this manual and online at www.nidekmedical.com under the ‘Maintenance Log’ tab.
7.Reinstall the access panel.
4.1.5 Wick Replacement
The wick is installed in the process air stream of the unit, connected to the air tank, to assist with removal of moisture from the air. This helps prevent premature contamination of the sieve beds, particularly in humid climates. The wick should be replaced at each compressor service / module replacement; it is suggested that more frequent replacement be done in very humid climates. The wick is located in a bushing located just to the right of the gearmotor. It is installed in a quick release fitting. Pull the fitting from the bushing by hand – it may require several pounds of force, remove the wick and install a new one in its place. Re-insert the wick end and fitting into the bushing so the wick is totally below the base plate surface.
|
Page 14 of 59 |
2010-2225 Rev — B |
4.1.6 Recording Maintenance
As the Home Service Provider, it is suggested that you record all routine maintenance and repairs performed on the Mark5 Nuvo® unit, including hours and dates of service in Appendix 15 of this manual and online at www.nidekmedical.com under the ‘Maintenance Log’ tab.
4.2Cleaning and Infection Control
With the growing concern about possible cross infection from home oxygen equipment (i.e.. oxygen concentrators) from one home care patient to another, appropriate cautions should be exercised.
The organisms of most concern are M. Tuberculosis, HIV, and Viral Hepatitis. These are potentially pathogenic.
Tuberculosis can survive outside of the human body, but its mode of transmission is by droplet nuclei. When infected individuals cough, they release droplet nuclei into the air, and these carry the Tuberculosis organism. Another person may breathe in these droplet nuclei, but prolonged exposure to the infected person is usually necessary for infection to occur.
HIV and Viral Hepatitis are both viruses, which are not living cells themselves but which can duplicate when in a living “host” cell. Both of these organisms are usually passed on by person-to-person contact, and both need to be in the human body to survive. Once outside the body, viruses can survive for only a short period of time.
4.2.1 Preparing for New Patient Use
When you remove the Mark5 Nuvo® from a patient’s home, always dispose of the used nasal cannula and humidifier bottle. Clean the exterior of the Mark5 Nuvo® with a soapy water solution or commercial detergent cleaner to remove any gross debris, organic or otherwise. Be careful not to get any liquid into the interior of the unit.
Next, clean the exterior with either a common chemical disinfectant or a bleach solution* and allow it to air dry. For the bleach solution, wear eye and skin protection to prevent exposure to the chlorine. Retest the Mark5 Nuvo® before you return it to your inventory.
Replace the cabinet air filter between each patient’s use or clean with warm soapy water if it is in good condition. Clean this filter at least once per week or more frequently if operated in a dusty environment.
*Make the bleach solution a 1:100 dilution of 5.25% sodium hypochlorite. Mix one part household bleach (e.g. Clorox) with 99 parts cold tap water. To measure the
|
2010-2225 Rev — B |
Page 15 of 59 |
solution easily, take 1/4 cup of household bleach, and mix it with a gallon of cold tap water. Allow the mixture to sit on potentially contaminated surfaces for 10 minutes.
5.0Service
5.1Components
The design of the Nidek Medical Mark5 Nuvo® Oxygen Concentrator allows for easy access and removal of most components. This allows you to perform scheduled maintenance, repair, and replacement of parts with minimal time and effort. The inlet air filter and battery are conveniently located inside the small access panel in the cabinet back.
CAUTION:
For your safety, be sure to set the I/0 (ON/OFF) switch to the 0 (OFF) position and unplug the power cord before you service the Mark5 Nuvo® Oxygen Concentrator.
NOTE: Record all scheduled maintenance. (Refer to Section 4.1.6.)
5.2Cabinet Removal
5.2.1 Removing Cabinet Back
To remove the cabinet back, remove two screws near the base (one on each side) and two inset screws at the top just under the handle.
5.2.2 Removing Cabinet Front
To remove the cabinet front, remove two screws near the base (one on each side) adjacent to the rear cabinet screws and two inset screws at the top just under the handle. It is necessary to remove the cabinet back to access the top screws.
5.2.3 Removing Control Panel
Eight screws hold the control panel in place; the front cover must be removed to access these screws. It is necessary to remove the electrical wiring and pneumatic tubing to fully release the control panel.
5.2.4 Main Structure
The weight and forces of the internal components rest solely on four parts: the main structure, the compressor plate, the base-plate and the base. These parts were specially designed and formed. They should never require replacement under normal use.
|
Page 16 of 59 |
2010-2225 Rev — B |
5.2.5 Caster Replacement
The casters are a push in type that does not require any fastener. Lay the device on its back to access the casters from the bottom. Pull them straight out away from the bottom.
5.3Compressor
The compressor is the pump within the oxygen concentrator that supplies air to the separation process performed by the sieve beds. The pressure generated by the compressor forces oxygen to flow out of the top of the sieve columns.
The compressor is the likely cause of two potential specific problems:
a.An insufficient amount of air is supplied to the process, and
b.An excessive sound level.
● Air Supply
Compressor output refers to how much compressed air the compressor can produce. This depends upon the model of the compressor, length of stroke, piston diameter, speed of rotation and condition of seals. The cup seals form the seal between the piston and the cylinder wall. As the cup seals wear, the output begins to gradually decrease. This reduction in compressor output results in less air, and thus less oxygen, entering the sieve beds. Therefore, the production of oxygen decreases.
Because this drop in oxygen production occurs over a long period of time, preventive maintenance on the compressor is not required.
You can continue a patient’s therapy on the Mark5 Nuvo® unit as long as the oxygen concentration level at the prescribed liter flow rate is within Nidek Medical’s specification limits. Refer to Section 2.4.
|
2010-2225 Rev — B |
Page 17 of 59 |

|
_______ |
Air Flow |
|
|
_______ |
Meter |
|
|
_______ |
||
|
_______ |
0 to 100 l/min |
|
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
1 bar |
|
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
Pressure |
|
|
_______ |
||
|
_______ |
||
|
_______ |
Gauge |
|
|
_______ |
||
|
_______ |
Pressure |
|
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
||
|
_______ |
Restrictor
Air
Filter
● Sound Level
The sound level is largely determined by the condition of the compressor’s bearings.
There are four bearings located within the compressor that allow the inner components of the compressor to rotate. If the bearings wear to the point that they become loose and noisy, the compressor becomes noticeably loud and needs servicing. The life of a compressor is determined primarily by its operating temperature. It is extremely important that the cabinet air filters are cleaned and replaced as required.
5.3.1 Compressor Replacement
Remove Compressor Assembly
To remove the compressor assembly for exchange, follow the steps listed below:
1.Set the unit’s I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2.Remove the cabinet back and front.
3.Disconnect the suction tube.
4.Disconnect the two compressor power cable leads and the two leads to the capacitor at the inline connector.
5.Remove the three screws that connect the compressor plate to the base of the unit, lift and slide out the compressor assembly.
6.Remove compressor from the plate by removing the four compressor bolts.
7.Remove tie wrap from compressor plate wiring harness.
8.Remove the heat exchanger tubing and fittings.
|
Page 18 of 59 |
2010-2225 Rev — B |
-
Contents
-
Table of Contents
-
Troubleshooting
-
Bookmarks
Quick Links
USER’S GUIDE
Mark 5 Nuvo (Std)
LITE
OXYGEN CONCENTRATOR
[Original language is English ]
Federal Law (US) restricts this device to
sale or use by, or on the order of, a licensed
physician. This oxygen concentrator should
be used only under the supervision of a
licensed physician.
I.
DESCRIPTION ……………………………………………..2
II.
III.1. Cleaning ………………………………………………………….4
III.3. Maintenance…………………………………………………….4
: ON (power switched on)
: Off (power switched off).
: Type B device
: Class II protection
: Do not expose to open ames.
2010-8400 Rev F
Danger: Do not smoke when using oxygen
CONTENTS
IV.
IV.7. Standards…………………………………………………………6
GLOSSARY OF SYMBOLS
: Do not use oil or grease.
: Technical information.
: Consult the accompanying documents.
: Keep in the vertical position.
: Fragile — handle with care.
May 2011
or when near this device.
1
Summary of Contents for Nidek Medical Mark 5 Nuvo LITE
Mark 5 Nuvo Lite — самый легкий кислородный концентратор в своем классе, а произведён он в США! При весе в 13,6 кг он производит до 5 литров О2 в минуту. Этот показатель крайне важен для лечения тяжёлых поражений органов дыхания. Модель предназначена для проведения терапии в санаториях, стационарах, ветеринарных клиниках, в случаях неотложной помощи и самое главное — в домашних условиях. Сам процесс работы аппарата «Марк 5 Нуво Лайт» происходит путём разделения газов, пропуская воздушный поток через адсорбенты, а также в этот момент осуществляется его тройная очистка.
Практически полную стерильность обеспечивают пылезащитная фильтрация входящего воздуха, фильтрующая кассета на входе в компрессор и бактериальный фильтр перед выпускным патрубком (<0,3 мкн).
Кроме лечения серьезных заболеваний дыхательных путей, концентратор кислорода «Mark 5 Nuvo Lite» можно применять с целью профилактики этих болезней — для приготовления коктейлей. Малые габариты и незначительный вес позволят без труда поместить его в палате медучреждения или дома, а в случае необходимости можно взять с собой в поездку. Ударопрочный корпус защитит от внешних повреждений.
Удобство эксплуатации — это также немаловажный фактор! Модель «Марк 5 Нуво Лайт» снабжена колесиками в нижней части корпуса и прочной ручкой, что позволяет без труда перемещать ее в нужное для вас место: так пожилые люди не испытывают дискомфорт во время терапии. При перебоях с электричеством или когда концентрация кислорода падает ниже 83%, вы услышите характерный звук. Эта функция дает возможность оперативно устранить проблему.
Приобретая концентратор кислорода Mark 5 Nuvo Lite, вы можете быть уверены в высоком качестве товара! В свою очередь мы выполняем гарантийное и постгарантийное обслуживание.
ОСОБЕННОСТИ МОДЕЛИ:
- Сигнализация падения концентрации кислорода ниже 83%.
- Антибактериальный фильтр.
- Сигнализация отключения сети.
- Счётчик времени работы аппарата.
- Ударопрочный корпус.
- Передвижной и маневренный.
КОМПЛЕКТ ПОСТАВКИ:
- Концентратор — 1 шт.
- Увлажнитель многоразовый — 1 шт.
- Назальная канюля, 2 м. — 1 шт.
- Кислородный шланг — 1 шт.
- Фильтр грубой очистки — 1 шт.
- Внутренний воздушный фильтр — 1 шт.
- Бактериальный фильтр — 1 шт.
- Руководство по эксплуатации — 1 шт.
- Гарантийный талон — 1 шт.
ИНФОРМАЦИЯ О ПРОИЗВОДИТЕЛЕ И ГАРАНТИИ:
- Гарантия 3 года.
- Наименование производителя: Nidek Medical Products Inc.
- Страна производства: США.

SERVICE MANUAL
Mark 5 Nuvo® Lite Family
(Nuvo Lite and Nuvo Lite 3)
For models: 520, 525, 535, 920, 925, and 935
And serial numbers beginning 132-XXXXX
3949 Valley East Industrial Drive Birmingham, Alabama 35217 USA
Telephone: (205) 856-7200 • 24-Hour Fax: (205) 856-0533
Nidek Medical is a trademark of Nidek Medical Products, Inc. Mark 5 Nuvo® is a registered trademark of Nidek Medical Products, Inc
|
2010-8407 Rev E |
April 30, 2018 |
Page 1 of 35 |
|
Contents |
|||
|
1.0 |
General Safety Warnings………………………………………………………………………………………………………………………. |
4 |
|
|
2.0 |
Glossary of Symbols …………………………………………………………………………………………………………………………….. |
5 |
|
|
3.0 |
About the Mark 5 Nuvo Lite Family ……………………………………………………………………………………………………….. |
6 |
|
|
3.1 |
Intended Use and Operation …………………………………………………………………………………………………………….. |
6 |
|
|
3.2 |
Installation and Storage ……………………………………………………………………………………………………………………. |
6 |
|
|
3.3 |
Alarms and Safety Features ………………………………………………………………………………………………………………. |
7 |
|
|
3.4 |
Device Performance and Specifications………………………………………………………………………………………………. |
8 |
|
|
4.0 |
Service Provider (Home / Clinic / Hospital)……………………………………………………………………………………………… |
9 |
|
|
4.1 |
Responsibilities ……………………………………………………………………………………………………………………………….. |
9 |
|
|
4.2 |
Operational Check……………………………………………………………………………………………………………………………. |
9 |
|
|
4.3 |
Patient / Caregiver Instruction…………………………………………………………………………………………………………. |
11 |
|
|
5.0 |
Service Technicians ……………………………………………………………………………………………………………………………. |
11 |
|
|
5.1 |
Testing and Troubleshooting …………………………………………………………………………………………………………… |
11 |
|
|
5.2 |
Troubleshooting Chart ……………………………………………………………………………………………………………………. |
15 |
|
|
5.3 |
Component Removal / Replacement Instruction ……………………………………………………………………………….. |
16 |
|
|
5.3.1 |
Remove Cabinet Back ………………………………………………………………………………………………………………… |
16 |
|
|
5.3.2 |
Remove Caster(s)………………………………………………………………………………………………………………………. |
16 |
|
|
5.3.3 Replace Inlet / Silencer Filter ………………………………………………………………………………………………………. |
17 |
||
|
5.3.4 Replace Final Product Filter ………………………………………………………………………………………………………… |
17 |
||
|
5.3.5 |
Replace Compressor ………………………………………………………………………………………………………………….. |
17 |
|
|
5.3.6 |
Replace Capacitor ……………………………………………………………………………………………………………………… |
18 |
|
|
5.3.7 |
Replace Control Valve………………………………………………………………………………………………………………… |
18 |
|
|
5.3.8 |
Replace Sieve Module………………………………………………………………………………………………………………… |
18 |
|
|
5.3.9 |
Replace Cabinet Fan…………………………………………………………………………………………………………………… |
19 |
|
2010-8407 Rev E |
April 30, 2018 |
Page 2 of 35 |
|
5.3.10 |
Replace Circuit Board (OCSI and Pressure) ………………………………………………………………………………… |
19 |
|
|
5.3.11 |
Adjust Regulator …………………………………………………………………………………………………………………….. |
20 |
|
|
5.3.12 |
Clean / Rebuild Regulator………………………………………………………………………………………………………… |
20 |
|
|
5.3.13 |
Replace Circuit Breaker …………………………………………………………………………………………………………… |
21 |
|
|
5.3.14 |
Replace Power Switch …………………………………………………………………………………………………………….. |
21 |
|
|
5.3.15 |
Replace Buzzer ………………………………………………………………………………………………………………………. |
21 |
|
|
5.3.16 |
Replace Hour Meter ……………………………………………………………………………………………………………….. |
21 |
|
|
5.3.17 |
Replace Flow Valve…………………………………………………………………………………………………………………. |
22 |
|
|
5.3.18 |
Replace Power Cord ……………………………………………………………………………………………………………….. |
22 |
|
|
5.4 |
Tools Required — Test Equipment / Gauges Available………………………………………………………………………….. |
22 |
|
|
6.0 |
Schematics / Assembly Drawings / Part Callouts……………………………………………………………………………………. |
23 |
|
|
6.1 |
Flow Schematic (OCSI models) ………………………………………………………………………………………………………… |
23 |
|
|
6.2 |
Flow Schematic (Standard models) ………………………………………………………………………………………………….. |
23 |
|
|
6.3 |
Electrical Schematic (all models) ……………………………………………………………………………………………………… |
24 |
|
|
6.4 |
Compressor Assembly / Parts Callout……………………………………………………………………………………………….. |
25 |
|
|
6.5 |
Front Cabinet Assembly / Parts Callout …………………………………………………………………………………………….. |
27 |
|
|
6.6 |
Back Cabinet Assembly / Parts Callout ……………………………………………………………………………………………… |
29 |
|
|
6.5 |
Module Assembly / Parts Callout……………………………………………………………………………………………………… |
31 |
|
|
Appendix A |
Service and Maintenance Log ………………………………………………………………………………………………….. |
33 |
|
|
Conformity with EN 60601-1………………………………………………………………………………………………………………………….. |
35 |
|
2010-8407 Rev E |
April 30, 2018 |
Page 3 of 35 |

1.0 General Safety Warnings
This unit is not a life-support device. Geriatric, pediatric, or any other patient unable to communicate discomfort while using this device should receive additional monitoring.
This device supplies highly concentrated oxygen enriched product gas that promotes rapid burning.
DO NOT allow smoking or open flames within the same room of this device or the administration accessory (cannula).
Failure to observe this warning can result in severe fire, property damage, and / or cause physical injury or death.
Oxygen accelerates the combustion of flammable substances.
DO NOT use oil, grease, petroleum based or other flammable products on the device, the administration accessory (cannula) or the patient’s face / neck.
Only persons who have read and understood this entire manual should be allowed to service the device.
CONTRAINDICATIONS — Those who continue to smoke (because of the increased fire risk and the probability that the poorer prognosis by smoking will offset the treatment benefit).
Federal Law (US) restricts this device to sale by, or on the order of, a licensed
physician. This oxygen concentrator should be used only under the supervision
Only
of a licensed physician.
|
2010-8407 Rev E |
April 30, 2018 |
Page 4 of 35 |
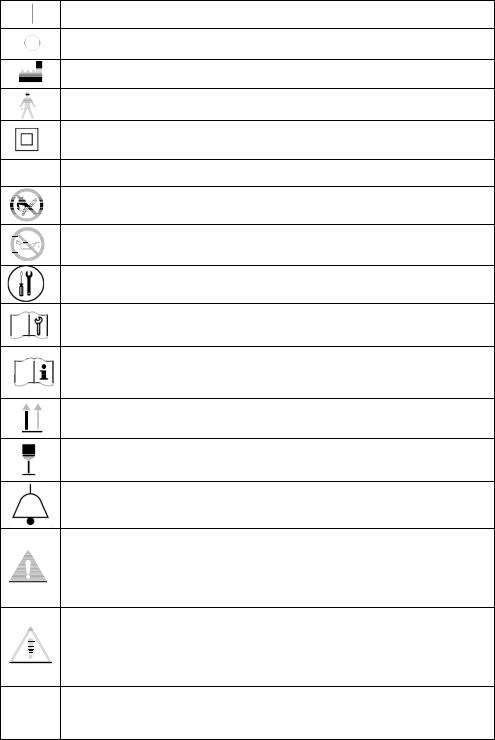
2.0 Glossary of Symbols
ON (Power switched on)
OFF (Power switched off)
Manufacturer Name and Address
Type B Device
Class II Protection
IPX1 Protection from vertically falling water drops
Do Not Expose to Open Flames
Do Not Expose to Oil or Grease
Tools Required / Technician Only
Refer to Technical Information / Service Manual
Refer to Instructions for Use / User’s Guide
Keep in Vertical Position
FRAGILE – Handle with Care
Visual Alarm Indicator
WARNING – A hazard or unsafe practice that can result in serious injury or death if conditions are not avoided.
Caution — A hazard or unsafe practice that can result in minor injury and / or property damage if conditions are not avoided.
Note – Information important enough to emphasize or repeat
|
2010-8407 Rev E |
April 30, 2018 |
Page 5 of 35 |

3.0About the Mark 5 Nuvo Lite Family
3.1Intended Use and Operation
The Mark 5 Nuvo Lite Family (Nuvo Lite and Nuvo Lite 3) Oxygen Concentrators are used as a means of providing continuous oxygen enriched product gas for patients, adolescent to geriatric, suffering from health conditions that cause low levels of oxygen in the blood (hypoxaemia).
The Mark 5 Nuvo Lite Family begins their operation with air being pulled into the external air intake filter. This filtered air enters the compressor via a suction resonator and fine filter. Pressurized air then exits the compressor and passes through a heat exchanger, which reduces the temperature of the compressed air. Next, an electronic valve system directs the air into one of two tubes that contain molecular sieve (sieve beds). The molecular sieve adsorbs (physically attracts) the nitrogen from the air as it is pushed through the sieve beds. This allows the oxygen enriched product gas to pass through before being delivered to the pressure regulator. As one tube is generating the product gas, the other is being purged of the adsorbed nitrogen, this process is called pressure swing adsorption (PSA). After passing through the regulator, the rate of product gas being delivered to the patient is set by the flow meter adjusting valve. Finally, it passes through a fine particle filter and then over a sensor that detects the oxygen concentration of the product gas before it exits the device through a fire resistant outlet.
Make sure during operation and after shut down that the cannula is facing away from soft surfaces and clothing. Excess oxygen can accumulate and cause ignition if exposed to a spark or open flame.
Use the power cord provided.
Check that the electrical characteristics of the power outlet used match those indicated on the manufacturer’s technical label on the rear panel of the device.
This unit may be equipped with a polarized plug. That is one blade wider than the other. If it does not fit into the outlet, reverse the plug. If it still does not fit, contact a qualified electrician. Do not defeat this safety feature.
3.2 Installation and Storage
The device should be operated in a dry area, with an ambient temperature between 10°C to 40°C (50°F to 105°F) at 15-95% relative humidity. The device can be operated at an altitude of up to 2200m (7500ft) at a temperature of 21°C (70°F) without causing product degradation.
DO NOT use in explosive atmosphere.
To avoid risk of fire and explosion the concentrator should be kept away from heat sources, incandescent sources, solvents, Aerosols, etc.
Unit should be placed and operated in a well-ventilated space that is free of pollutants or fumes and protected from the elements with adequate lighting.
Unit should be placed and operated in a space where the position and storage of the mains cable and oxygen tubing do not present a tripping hazard.
The mains cable should be easily accessible for disconnection.
For patient safety and benefit, no modification to the equipment is allowed.
It is also not recommended to interconnect the device with any equipment or accessories not specified in this guide.
Device must have power to operate.
In the event of power loss and for continued operation a backup source is recommended.
Do not use in a specifically magnetic environment (MRI, X-ray, etc.). May cause device malfunction.
We recommend against the use of extension cords and adapters, as they are potential sources of sparks and fire.
|
2010-8407 Rev E |
April 30, 2018 |
Page 6 of 35 |

Consult your equipment provider for further information regarding altitudes of 2200 m to 4000m (7500 to 13000ft).
The device should be stored in a dry area, with an ambient temperature between -20°C to 60°C (0°F to 140°F) at 1595% relative humidity. It must be stored, transported and used in the vertical position only.
Oxygen concentration can be affected after prolonged periods of storage – check device before use.
3.3 Alarms and Safety Features
Each device is equipped with indicator lights (green and yellow) and auditory indicators to identify various operational modes. Devices manufactured prior to 2018 were equipped with red and yellow indicator lights. The alarm modes are described below:
The device has an audible alarm to warn the user of problems. In order that the alarm may be heard, the maximum distance that the user can move away from it must be determined to suit the surrounding noise level.
If any of the below alarm conditions occur, press the Power Switch to the “O” (OFF) position. Refer to the Troubleshooting Guide in §5.2 for the possible cause and solution.
No voltage detection: In the event of a loss of mains power, an intermittent audible alarm is activated and the green light is no longer illuminated.
Oxygen Concentration Status Indicator: If supplied, in the event that the oxygen concentration falls below the set point percentage, a continuous audible alarm and the yellow indicator light will actuate. The oxygen concentration monitor is an electronic module capable of checking the effective oxygen concentration supplied by the concentrator. When the device is started, the green indicator light will flash until the concentration set point is reached (approximately two minutes).
A solid green indicator light means the power is applied to the concentrator and that it is ready to provide oxygen enriched air to the patient.
No special maintenance is required. The alarm set-point is factory set and the setting cannot be adjusted. All OCSI models are set at 85% ± 3%.
Blocked Cannula detection: If supplied, the device has a Blockage Alarm. A continuous audible alarm and both indicator lights will be lit immediately in the event the flow of oxygen to patient becomes blocked.
Malfunction detection: If low pressure occurs due to a mechanical failure, the indicator light will flash yellow and a continuous audible alarm will actuate.
Thermal safety: The compressor motor is protected by a thermal switch situated in the stator winding (145 ± 5° C). One tubeaxial fan cools the compressor compartment.
Electrical protection:
•A 5A circuit breaker is incorporated into the front cabinet of all 230V models
•A 10A circuit breaker is incorporated into the front cabinet of all 115V models
•Class II devices with insulated casings (EN60601-1 standard)
Safety valve: This is fitted on the compressor outlet and is calibrated to 3.4 bar (50 psig).
Fire Break: This device is fitted with a metal fire break at the Oxygen Product Outlet. This break will keep fire from entering the device.
|
2010-8407 Rev E |
April 30, 2018 |
Page 7 of 35 |

3.4 Device Performance and Specifications
The performance of the device (especially the oxygen concentration) is quoted at 21°C (70°F) and one atmosphere. The specifications may change with temperature and altitude.
|
Model |
520 |
525 |
920 |
925 |
535 |
935 |
||
|
Description |
5 LPM 115V |
5 LPM 230V |
3 LPM |
3 LPM |
||||
|
115V |
230V |
|||||||
|
Frequency |
60 Hz |
50 Hz |
60Hz |
50 Hz |
||||
|
Average Power |
330 Watts |
300 Watts |
210 |
180 |
||||
|
Watts |
Watts |
|||||||
|
Protection Class |
Class II |
|||||||
|
Mains Protection |
10A |
5A |
5A |
|||||
|
Average Oxygen Content |
At 2 LPM |
|||||||
|
> 90% |
||||||||
|
Average Oxygen Content |
At 5 LPM |
At 3 LPM |
||||||
|
87% to 95.5% |
87% to 95.5% |
|||||||
|
Liter Flow |
0.125 to 5 LPM |
0.125 to 3 LPM |
||||||
|
Outlet Pressure |
7 Psig |
|||||||
|
Dimensions |
||||||||
|
(L x W x H) |
36 x 23 x 58.5 cm (14 x 9 x 23 in.) |
|||||||
|
Weight |
14.5 kg (32 lbs.)* |
|||||||
|
Noise Level |
< 58 dBA |
|||||||
*Weight dependent on model and features
In compliance with EN ISO 80601-2-69, the flow supplied is equal to the flow set on the flowmeter, accurate to within ± 10% or 200 ml/min, whichever is greater.
The variation of the maximum recommended flow does not exceed ± 10 % of the indicated value when a back pressure of 6.9 kPa (1 psig) is applied to the output of the device.
Complies with EN 60529:2001 + A2:2014 rating of IPX1; enclosure protects internal electrical components against vertically falling water drops.
Complies with EN 60601-1:2006 [11.6.3]; enclosure protects internal electrical components against spilling of a glass of water (i.e. contents of humidifier).
|
2010-8407 Rev E |
April 30, 2018 |
Page 8 of 35 |

4.0Service Provider (Home / Clinic / Hospital)
4.1Responsibilities
Service Providers of the Mark 5 Nuvo Lite Family (Nuvo Lite and Nuvo Lite 3) Oxygen Concentrators must assume responsibilities for handling, operational testing, patient instruction, and maintenance. These responsibilities are outlined below and throughout this manual.
To ensure patient safety, use only after one or more settings have been individually determined or prescribed for the patient at their specific activity levels – AND – only use the accessories that were used when the settings were determined.
While undergoing oxygen therapy, if the patient feels discomfort or experiences a medical emergency, seek medical assistance immediately.
As a Service Provider, you should do all of the following:
•Inspect the condition of each device immediately upon delivery to your location. Note any sign of damage, on the delivery receipt, and immediately report it directly to both the freight company and Nidek Medical Products, Inc.
•Ensure the operation of each device before delivery to a patient by completing the “Operational Check” provided in §4.2.
•Ensure each device has been thoroughly cleaned, the cabinet filter has been cleaned or replaced, and the nasal cannula and tubing has been replaced before delivering the device to a new patient or between patients.
•Deliver device only to patients authorized by a physician’s prescription. The device must not be used as a life-supporting or life sustaining device.
•Ensure a backup supply of oxygen is available.
•Instruct patients and patient caregivers how to use the device in conjunction with the Instructions for Use (PN 2010-8401CE), including required routine maintenance and cleaning of the device and filters. See “Patient / Caregiver Instruction” provided in §4.3.
•Record and notify Nidek Medical of any and all complaints provided by the patient or patient caregiver.
oThe FDA defines a complaint as “any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or performance of a device after it is released for distribution.”
•Be available to service each patient at any time.
•Keep service records of each device. A form approved by Nidek Medical has been provided in Appendix A.
•Repair components and replace parts only as outlined in this manual. Use only Nidek Medical parts for replacement. Refer to the Product Warranty Statement if parts replacement is required within the warranty period.
4.2Operational Check
Nidek Medical runs each device through a burn in period and tests every device thoroughly after manufacture before releasing it for shipment. As the Service Provider, it is your responsibility to perform the following check to ensure that the device is operational.
|
2010-8407 Rev E |
April 30, 2018 |
Page 9 of 35 |
1.Plug in the power cord of the unit, following the electrical characteristics noted on the technical label, and set the Power Switch to the I (ON) position. The compressor should begin running.
2.Once the device has been running for approximately ten minutes, verify the concentration is within specifications, the flow is accurate and the alarms are operational. For units equipped with the Oxygen
Concentration Status Indicator (OCSI) (models 525, 535, 925, 935), the green indicator light will flash until the oxygen concentration reaches 85% ± 3% (approximately two minutes).
a.Verify oxygen concentration by:
i.Connect a calibrated oxygen concentration analyzer to the oxygen outlet.
1.If an oxygen humidifier bottle is used, remove it from the oxygen outlet. The concentration readings of the product gas after it has passed through a humidifier bottle will be erroneous and can result in damage to your analyzer.
2.Oxygen analyzers are available for purchase from Nidek Medical (see §5.4 for testing equipment part numbers).
ii.Set the flow rate of the device to the prescribed rate of the patient.
iii.Record oxygen concentration readings over a period of several minutes to reduce any cyclic variations. These readings should fall within the specifications provided in §3.4.
1.If not, refer to the troubleshooting chart in §5.2 for possible causes and solutions.
b.Verify the flow is accurate by:
i.Connect a calibrated flow gauge to the oxygen outlet.
1.If an oxygen humidifier bottle is used, remove it from the oxygen outlet.
2.Gauges are available for purchase from Nidek Medical (see §5.4 for testing equipment part numbers).
ii.Turn the flow knob clockwise until it stops (wide open).
iii.The reading should indicate 5 liters/min. (models 520, 525, 920 and 925) or 3 liters/min (models 535 and 935).
1.If not, refer to §5.3.11 for adjusting the regulator, or refer to the troubleshooting chart in §5.2 for other possible causes and solutions.
c.Verify alarms are operational by:
i.No Voltage Alarm
1.Unplug the power cord from the wall outlet.
2.Set the Power Switch to the I (ON) position.
3.This should immediately activate the intermittent audible alarm and there should be no indicator lights actuated.
a.If it does not, refer to the troubleshooting chart in §5.2 for possible causes and solutions.
ii.Blockage Alarm
1.Adjust the Flow Knob to desired flow rate.
2.Block the oxygen flow at the patient outlet
3.This should immediately activate a continuous audible alarm and both indicator lights should be actuated.
a.If it does not, refer to the troubleshooting chart in §5.2 for possible causes and solutions.
|
2010-8407 Rev E |
April 30, 2018 |
Page 10 of 35 |

4.3 Patient / Caregiver Instruction
It is important that the patient and/or caregiver thoroughly understands how to operate their device. This enables proper treatment as prescribed by a qualified, licensed physician. You must explain that the purpose of this therapy is to alleviate symptoms. If the patient experiences any discomfort or the unit alarm sounds, they must notify their Service Provider and/or physician immediately. You, as the Service Provider, are responsible to see that each patient receives the Instructions for Use (IFU) (PN 2010-8401CE) and understands how to operate, clean and maintain their device.
•Refer to §4.2 in the IFU for the Start-Up procedure.
•Refer to §4.3 in the IFU for the Shut Down procedure.
•Refer to §5.1 in the IFU for the Cleaning procedures of the device itself, the filters and the accessories.
•Refer to §5.2 in the IFU for the Maintenance procedure.
Refer to §5.3.3 and §5.3.4 of this manual for instruction on replacing the inlet and final product filters.
5.0 Service Technicians
The design of the Mark 5 Nuvo Lite Family (Nuvo Lite and Nuvo Lite 3) allows for easy access and removal of most components. This allows you to perform scheduled maintenance, repair, and replacement of parts with minimal time and effort.
•Refer to §5.2 for the Troubleshooting Chart for a list of problems, possible causes and solutions.
•Keep service records of each device. A form approved by Nidek Medical has been provided in Appendix A.
•Repair components and replace parts only as outlined in this manual. Use only Nidek Medical parts for replacement.
•Refer to the Product Warranty Statement if parts replacement is required within the warranty period.
•Analyzers and gauges are available for purchase from Nidek Medical, see §5.4 for part numbers.
For your safety, be sure to set the Power Switch to O (OFF) position and unplug the power cord before you service the device.
5.1 Testing and Troubleshooting
Before reviewing the troubleshooting chart, the following questions may be useful to isolate any malfunctions.
1.Does the concentrator turn on when the switch is activated?
2.Are the filters clean?
3.Connect an oxygen analyzer to the outlet fitting of the device. Set the flow to 2LPM, is the concentration greater than 90%? Set the flow at 5 LPM (or 3LPM for models 535 and 935), is the concentration between 87 and 95.5%?
4.Connect test pressure gauge to the outlet fitting of the unit. Does the pressure read 7.1 psig (49 kPa) ± 20%?
5.Perform an air pressure test (P1). Does the pressure cycle between 10-15 and 25-32 psig approximately (70103 and 172-220 kPa)?
a.Remove the upper cabinet back (see §5.3.1 for instructions).
b.Remove the air supply tubing going to the control valve and install the test port tee fitting. (See Figures 5.1.1 and 5.1.2 below for Normal Operating Configuration and Test Port Configuration.)
|
2010-8407 Rev E |
April 30, 2018 |
Page 11 of 35 |

Nidek Medical Mark 5 Nuvo Lite Family: List of Available Documents
Note for Owners:
Guidesimo.com webproject is not a service center of Nidek Medical trademark and does not carries out works for diagnosis and repair of faulty Nidek Medical Mark 5 Nuvo Lite Family equipment. For quality services, please contact an official service center of Nidek Medical company. On our website you can read and download documentation for your Nidek Medical Mark 5 Nuvo Lite Family device for free and familiarize yourself with the technical specifications of device.
More Medical Equipment Devices:
-
Omnigon Flexima 3S
1. Before applying the baseplate2. Preparing the skin3. Preparing the baseplate4. Clipping the pouch1A. Use a measuring guide to determine the size of your stoma. 1B. Cut the skin barrier to the measured size of your stoma.It is important to cut the baseplate to the right size and shape of your stoma. Remain within the maximum possible cutting line on the baseplate and allow about 2mm around yo …
Flexima 3S Medical Equipment, 2
-
Animas OneTouch Ping
Gather your supplies.Pump Infusion Set IV Prep and Alcohol Wipe (or 2 alcohol wipes)Cartridge Insulin VialHow to Change Your OneTouch Ping® Infusion Set and CartridgeWash your hands.Clean top of insulin vial with alcohol wipe.Page 1 of 21. Unscrew black cartridge cap from pump.2. Thread tubing connector through small end of black cartridge cap.3. Remove small, clear cap from cartridge.4. Screw t …
OneTouch Ping Medical Equipment, 2
-
Physio Control LIFEPAK 15
Title LIFEPAK 15 Monitor Defibrillator Temperature Upgrade Page 1 of 34 Doc Type Service Process Document Doc ID 3308212 Doc Rev C Doc State Released State Date 05/21/2019 Owner Corporate Attachments (Optional): Qty Pages Page Sequencing Change Description No changes to process or procedure. Documentation was updated to change Physio part numbers to catalog …
LIFEPAK 15 Medical Equipment, 34
-
PLUSOPTIX S16
Page 1 of 3 MS 08.06.2017 Plusoptix GmbH · Neumeyerstr. 48 · 90411 Nuremberg · Germany · www.plusoptix.com Software Update preparation plusoptiX S16 Prepare an empty USB stick with a minimum capacity of 2 GB. Click on the download-link with the newest software update which you can find on our homepage and save the file on your computer. You will need the following tools to ma …
S16 Medical Equipment, 3
Recommended Documentation:
Быстрая Доставка
Курьерская доставка заказов по всей России
- до Дверей
- до Терминала
Оплата при Получении
(наложенный платеж)
Оплата наличными или картой курьеру, после проверки соответствия, комплектации, работоспособности
Top 5 Товаров
Новости / Информация
Мы в социальных сетях

Эта модель доступна в аренду по Москве и Области
-
Описание
-
Устройство Mark 5 Nuvo Lite из линейки товаров американского бренда Nidek оптимально подойдет для оксигенотерапии на дому. Благодаря данному прибору лечение кислородной недостаточности является максимально простым и удобным для пациента. Преимуществом данной модели является минимальный вес, составляющий всего 13,6 кг. Корпус устройства снабжен ручкой и колесами, поэтому его перемещение не представляет сложности даже для пациентов зрелого возраста или людей с ограниченной подвижностью.
Компактный прибор занимает мало места, поэтому Mark 5 Nuvo Lite без труда устанавливается не только в помещении, но и в салоне медицинского транспорта. Аппарат отличается простотой настройки, а уровень шума при работе не создает неудобств. При этом модель является очень производительной, благодаря чему может успешно использоваться для профилактического лечения и оказания срочной медицинской помощи.Экономичность и легкость в обслуживании
Достоинством концентратора Mark 5 Nuvo Lite является простота сервисного обслуживания. При регулярном применении прибора замена фильтра предварительной очистки производится всего один раз в год.
В целях повышения эффективности кислородной терапии устройство оборудовано трехступенчатой системой фильтрации воздуха, благодаря чему происходит не только повышение содержания кислорода, но и бактериальная очистка воздуха.
Также Mark 5 Nuvo Lite снабжен диагностическим модулем для непрерывного контроля содержания кислорода. При изменении показателей срабатывает световой и звуковой индикатор.Применение устройства
Концентратор кислорода «Марк 5 НУВО Лайт» компактный и высокопроизводительный прибор.
«Марк 5 Нуво Лайт» используется очень широко:- Для организации лечения на дому с целью продолжительной кислородной терапии.
- В медицинских учреждениях, при проведении мер неотложной помощи.
- При проведении процедур профилактической кислородотерапии.
- Предназначен для длительной кислородотерапии на дому, в больничных и поликлинических условиях.
- Расходомер с регулятором потока кислорода от 0, 125 до 5 л/мин. (12 положений регулятора потока)
- При максимальном потоке 5 л/мин концентрация кислорода составляет более 90%.
- Круглосуточная работа в течение 24 часов.
- Звуковые предупреждения и оптические индикаторы.
- Счетчик проработанного времени (в часах).
В спортивных клубах и учреждениях санаторно-курортного типа прибор можно использовать для приготовления кислородных коктейлей.
ТЕХНИЧЕСКИЕ ХАРАКТЕРИСТИКИ Марк 5 НУВО Лайт
Ресурс до 30 000 часов Электропитание 220/230В ~50Гц 1,55А Поток кислорода от 0,125 до 5 литров в минуту (л/мин) Концентрация кислорода в процентах от 0,125 до 5 л/мин 90% (+6,5%/3%) Максимальное давление кислорода на выходе 50,0 кПа (7,0 psig) Подсчет проработанного времени Счетчик времени (в часах) Уровень шума менее < 45 ДБ Мощность максимум 290 Вт Принадлежности Увлажнитель кислорода
Канюля носовая с трубкой
Маска кислородная с трубкойЭксплуатация при температуре и влажности от 5°С до 40°С, от 40% до 95% Условия хранения Температура: от 40°С до 70°С
Влажность: от 10% до 100%Вес Нетто 14,5 кг Вес Брутто 16 кг Упаковка Марк 5 нуво лайт Картонная коробка с полиуретановыми вставками Габаритные размеры 585 мм (в) х 360 мм (ш) х 230 мм (г) Габаритные размеры упаковки 660 мм (в) х 480 мм (ш) х 340 мм (г) Соответствие европейским директивам и стандартам 93/42 EEC, IEC606012, EN60601+A1+A2,
ISO8359:1996, ISO9001: 2000/ISO13485:2003Классификация устройства класс II, тип B Гарантия 3 года -
Комплектация
-
Комплектация Nidek Марк 5 нуво лайт
Концентратор кислорода Nidek Mark 5 Nuvo Lite 1 шт
Удлинительная кислородная трубка 10 м. 1шт.
Маска кислородная 1шт.
Канюля кислородная, трубка 1.8м. 1 шт
Увлажнитель кислорода 1 шт
Фильтр грубой очистки 1 шт
Фильр тонкой очитки, оригинал для Mark 5 Nuvo Lite 1 шт
Инструкция по эксплуатации 1 шт
Гарантийный талон 1 шт
-
Документы
-
Инструкция по эксплуатации
-
Руководство по эксплуатации mark 5 nuvo lite инструкция
-
Техзадание
-
Техническое задание на Кислородный концентратор Mark 5 nuvo lite
Скачать в формате .docx
Комплект поставки:
Концентратор кислорода 1 шт.
Удлинительная кислородная трубка 10 м. 1шт.
Маска кислородная 1шт.
Канюля кислородная, трубка 1.8м. 1 шт.
Увлажнитель кислорода 1 шт.
Фильтр грубой очистки 1 шт.
Фильтр тонкой очитки 1 шт.
Инструкция по эксплуатации 1 шт.
Гарантийный талон 1 шт.Наименование товара, товарный знак Концентратор кислорода Mark 5 Nuvo Lite Наименование страны происхождения товара Соединенные Штаты Америки, ОКСМ: USA, 840 Описание требований Наличие функции или величина параметра Назначение Для длительной кислородотерапии Счетчик проработанного времени (в часах) наличие Звуковые предупреждения, оптические индикаторы наличие Оптические индикаторы наличие Уровень шума 37 Дб Максимальное давление кислорода на выходе 50,0 кПа Поток кислорода в диапазоне от 0,125 л/мин до 5 л/мин (12 позиций переключателя) Концентрация кислорода 90-95% Электропитание 220В, 50Гц, 1,55А Потребляемая электрическая мощность 290 Вт Габаритные размеры 580 мм (высота) х 350 мм (ширина) х 220 мм (глубина) Размеры в упаковке 660 мм (в) х 480 мм (ш) х 340 мм (г) Масса, нетто 13,6 кг Масса, брутто 16 кг Упаковка Картонная коробка Колесики для передвижения 4 шт. вмонтированы в корпус Классификация устройства класс II, тип B Гарантия производителя 3 года

Единиц товара в коробке: 1
Этот концентратор приобретался на замену вышедшему из строя Атмунг 5лН. Сравнивать есть с чем, отмечу плюсы При той же производительности,1.Размеры в два раза меньше 2.Значительно легче по весу 3.Тише работает, со слов консультанта и информации из инструкции на марк 5 нуво лайт, в нем встроен газоанализатор который сигнализирует о снижении концентрации кислорода на выходе (пока не срабатывал), что крайне важно для человека напрямую зависящего от концентратора. Подключение и настройка проще простого, всё в комплекте. Прибор рекомендую! заказ №0147
Заказ 127. С огромной благодарностью и наилучшими пожеланиями!
Концентратор приобретался для больного ХОБЛ. С помощью консультантов, выбор пал именно на эту модель за счет небольшого веса, габаритов и удобства пользования. Всё управление сводится к включению и выключению, пожилой человек с легкостью справляется. Используем концентратор Марк 5 уже вторую неделю, никаких нареканий нет, всё работает отлично.В комплекте есть всё необходимое, ничего не нужно докупать. Сотрудников магазина благодарим за консультацию, предоставленную скидку и быструю доставку.
Нет равных !!!
09.07.2018 года заказал Кислородный концентратор Mark 5 Nuvo Lite производстра и Сборки США! с 10% скидкой получилось 47 610 рублей. То есть по цене Китайских тракторов типа Амед или Амунг. Пульсоксиметр подарили бесплатно, доставили в Истру, на следующий день 10.07.2018 года, доставка бесплатная!!! Что тоже очень порадовало. Концентратор кислорода Mark 5 Nuvo Lite за месяц непрерывной (24 часа в сутки) эксплуатации показал себя превосходно: очень легки, очень тихий (по уровню шума как домашний компьютер) и ,самое главное в моем случае, очень простой!!! (покупался для пожилого человека 82 года ХОБЛ). Ребятам из Air-med.ru отдельное Спасибо! За прекрасную слаженную работу и отличные условия для покупки (бесплатная и быстрая доставка, скидка и подарки, проверка концентратора при доставке и обучение по его использованию). Успехов и процветания!!!
Другие отзывы
Вы уже оставили отзыв об этом товаре. Спасибо!
Mark 5 Nuvo Lite — самый легкий кислородный концентратор в своем классе, а произведён он в США! При весе в 13,6 кг он производит до 5 литров О2 в минуту. Этот показатель крайне важен для лечения тяжёлых поражений органов дыхания. Модель предназначена для проведения терапии в санаториях, стационарах, ветеринарных клиниках, в случаях неотложной помощи и самое главное — в домашних условиях. Сам процесс работы аппарата «Марк 5 Нуво Лайт» происходит путём разделения газов, пропуская воздушный поток через адсорбенты, а также в этот момент осуществляется его тройная очистка.
Практически полную стерильность обеспечивают пылезащитная фильтрация входящего воздуха, фильтрующая кассета на входе в компрессор и бактериальный фильтр перед выпускным патрубком (<0,3 мкн).
Кроме лечения серьезных заболеваний дыхательных путей, концентратор кислорода «Mark 5 Nuvo Lite» можно применять с целью профилактики этих болезней — для приготовления коктейлей. Малые габариты и незначительный вес позволят без труда поместить его в палате медучреждения или дома, а в случае необходимости можно взять с собой в поездку. Ударопрочный корпус защитит от внешних повреждений.
Удобство эксплуатации — это также немаловажный фактор! Модель «Марк 5 Нуво Лайт» снабжена колесиками в нижней части корпуса и прочной ручкой, что позволяет без труда перемещать ее в нужное для вас место: так пожилые люди не испытывают дискомфорт во время терапии. При перебоях с электричеством или когда концентрация кислорода падает ниже 83%, вы услышите характерный звук. Эта функция дает возможность оперативно устранить проблему.
Приобретая концентратор кислорода Mark 5 Nuvo Lite, вы можете быть уверены в высоком качестве товара! В свою очередь мы выполняем гарантийное и постгарантийное обслуживание.
ОСОБЕННОСТИ МОДЕЛИ:
- Сигнализация падения концентрации кислорода ниже 83%.
- Антибактериальный фильтр.
- Сигнализация отключения сети.
- Счётчик времени работы аппарата.
- Ударопрочный корпус.
- Передвижной и маневренный.
КОМПЛЕКТ ПОСТАВКИ:
- Концентратор — 1 шт.
- Увлажнитель многоразовый — 1 шт.
- Назальная канюля, 2 м. — 1 шт.
- Кислородный шланг — 1 шт.
- Фильтр грубой очистки — 1 шт.
- Внутренний воздушный фильтр — 1 шт.
- Бактериальный фильтр — 1 шт.
- Руководство по эксплуатации — 1 шт.
- Гарантийный талон — 1 шт.
ИНФОРМАЦИЯ О ПРОИЗВОДИТЕЛЕ И ГАРАНТИИ:
- Гарантия 3 года.
- Наименование производителя: Nidek Medical Products Inc.
- Страна производства: США.

